Research Spotlight: Modelling of Radiolytic Production of Nitric Acid
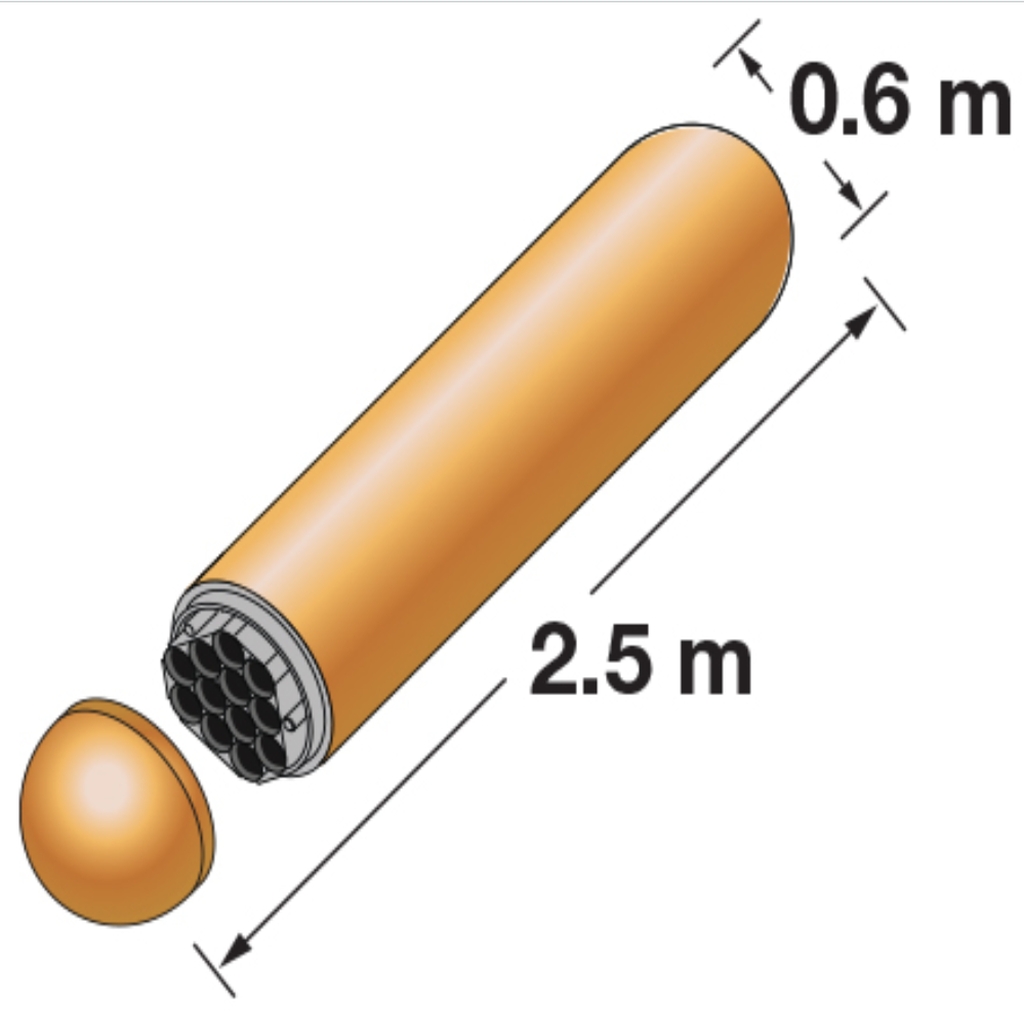
The long-term disposal of spent nuclear fuel is a pressing issue, and research is being carried out in many countries to devise a safe solution. Canada plans to store spent fuel in a deep geological repository with multiple barriers to prevent any radioactive materials from reaching the surface. A key barrier is the used fuel container (left image), which was recently redesigned. The current design consists of an inner vessel made of carbon steel for structural strength, with an outer coating of corrosion-resistant copper. In order to have high confidence in the safety of this design, it is necessary to be able to predict the results of various corrosion scenarios, even if they are unlikely. One such scenario is corrosion of the copper surface by reactive species such as H2O2 and HNO3 that could form in moisture exposed to radiation from the spent fuel. This work models the dose rates (energy received per unit mass per unit time) at the container surfaces as a function of fuel age, assuming a hemispherical volume for the water droplet (middle image). It uses a humid-air radiolysis model to study the effects of dose rate and humidity on radiolytic oxidant production for conditions where unexpected early water intrusion saturates the clay materials packed around the containers and reaches the copper metal surface.
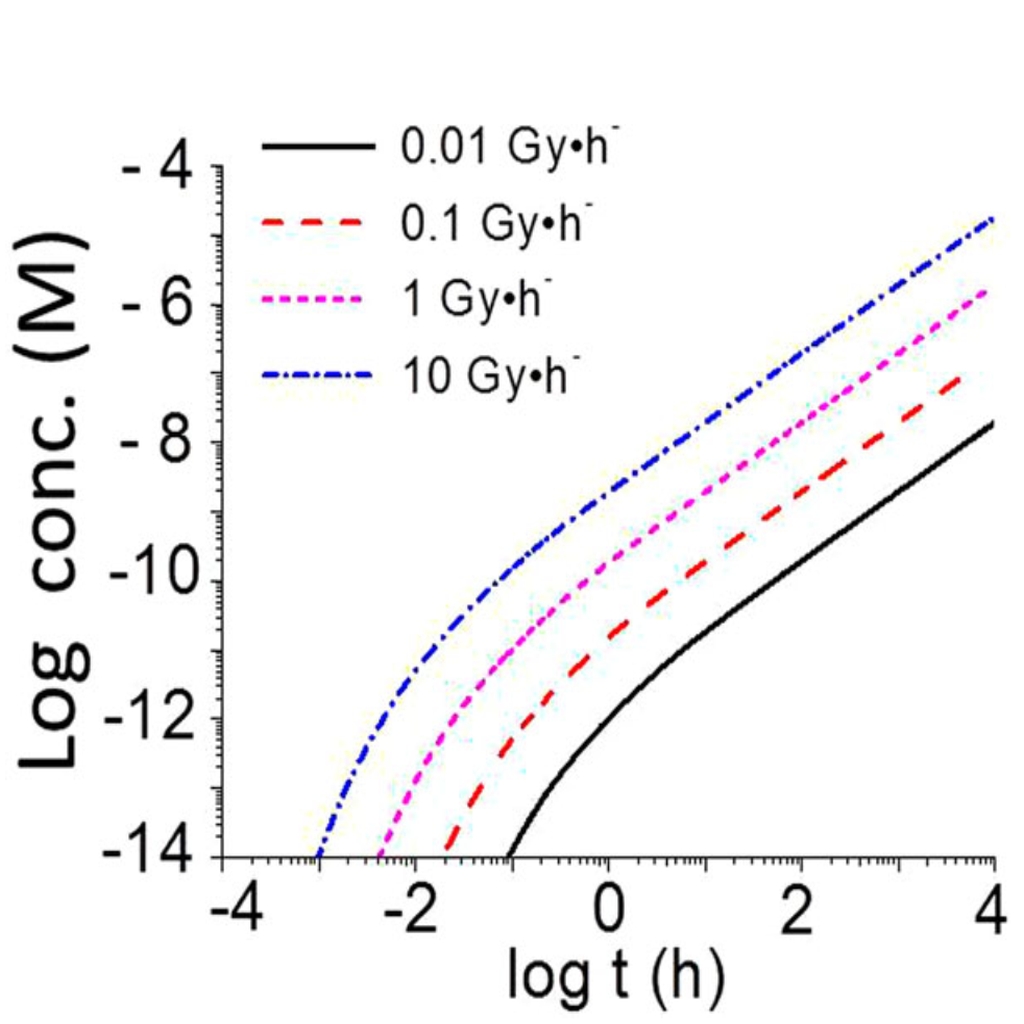
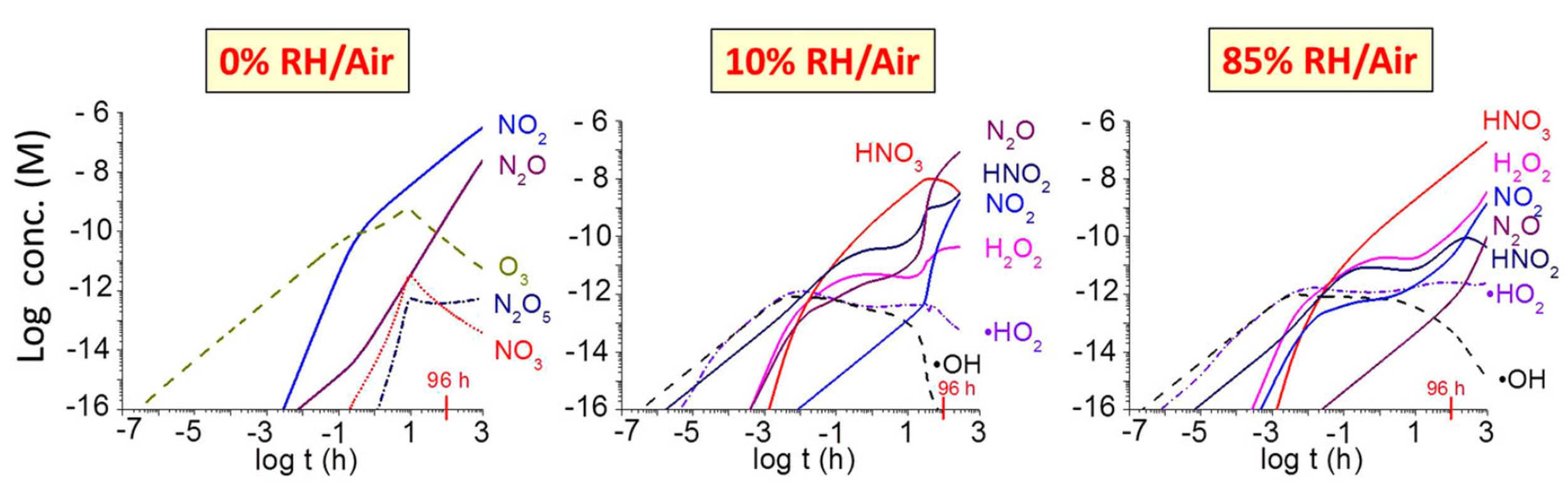
The mathematical model was formulated using the known thermodynamic and kinetic data for the system, and coded using modelling software. The graphs below show the results of the calculations for dry air (0% relative humidity ) and humid air (10% and 85% relative humidity). The graph above (right image) shows how the concentration of nitric acid produced depends on the dose rate.
When making predictions about the degradation of materials that are critical for safety, we usually do it conservatively. This means that we assume the worst for all the parameters, which should give an upper bound for the predicted extent of corrosion. The HNO3 production rate in a condensed water droplet formed on a container surface was conservatively estimated by assuming that every •OH, the main species produced by primary radiolytic processes, is immediately converted to HNO3 in the gas phase. We then assumed that all of this HNO3 is absorbed into the water droplet. The assumption was also made that all of the nitric acid absorbed in the water droplet is consumed in corroding copper uniformly. The results of the calculations predict corrosion to a depth of 9.4 μm over the permanent storage time. As the planned thickness of the copper coating on the used fuel container is 3-4 mm, this means that even if this scenario occurs, corrosion of the copper surface will only result in the loss of a very small fraction of the metal.
Canada's copper-coated used fuel containers are being subjected to a wide range of tests for their resistance to chemical and physical degradation. As in the study presented here, none of these studies have given any reason to doubt the safety of the used fuel container design.
Our publication on this work is:
Modelling of Radiolytic Production of HNO3 Relevant to Corrosion of a Used Fuel Container in Deep Geologic Repository Environments, R. P. Morco , J. M. Joseph, D. S. Hall , C. Medri , D. W. Shoesmith and J. C. Wren,
Corros. Eng. Sci Techn. 52(S1), 141-147 (2017)
And it can be found here (you may need to have access through your institution):
http://www.tandfonline.com/doi/full/10.1080/1478422X.2017.1340227